Spun out by researchers at the University of Melbourne, brain-computer interface company Synchron this week became the first company to begin clinical trials in the United States, ahead of Elon Musk’s Neuralink.
Synchron is trialing its Stentrode implant which aims to allow severely paralysed patients to control digital devices using their brain. The early feasibility study admitted its first patient at Mount Sinai Hospital in New York.
Synchron chief executive and co-founder Dr Thomas Oxley said the trials would be expanded to locations in Pittsburgh, Melbourne, Sydney, Brisbane and the Gold Coast in the coming weeks as the company aims to trial another 20 patients. He said the company would test the efficacy of Stentrode in preparation for a commercial product trial in at least a couple of years.
This follows the completion of a successful test on four patients in Melbourne, which was presented at the American Academy of Neurology last month. After a year of monitoring, the patients are now able to send text messages, conduct online shopping, manage their finances, and access telehealth services using the Stentrode system unsupervised.
When asked about comparisons to Elon Musk’s Neuralink, Synchron chief technology officer and co-founder Professor Nick Opie said his company has progressed further in terms of testing.
“We’ve finished a human clinical trial in Australia and we’re starting one in the US. To my knowledge the FDA has not approved Neuralink for a human trial, so in that regard I think, yes we are certainly leading this field,” Professor Opie said.
“Australia’s pioneering in a lot of fields of medicine, not just brain machine interfaces, there’s certainly a lot of amazing technology that is being conceptualised here or taken through to product here. [Stentrode] is just another example of Australia’s brilliance and global leadership in medical bionics translation.”
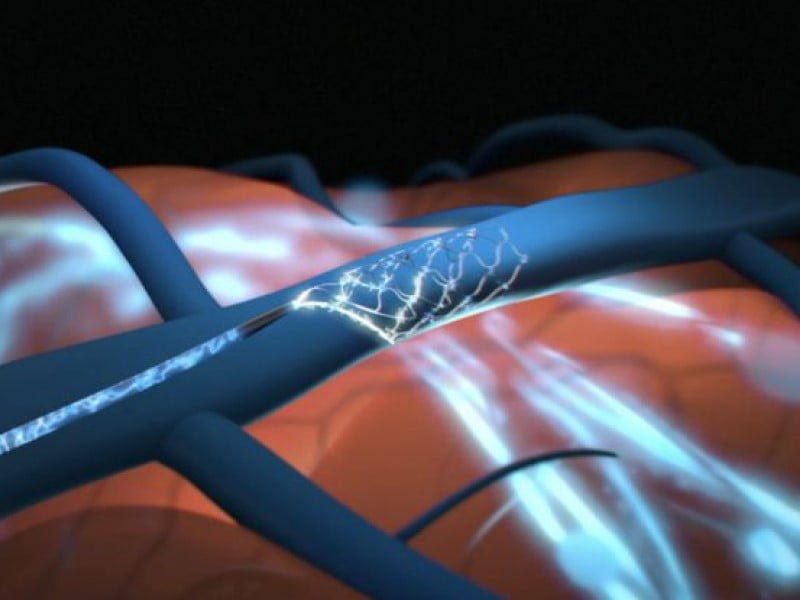
Stentrode converts brain activity into a standardised digital language, allowing patients to use their computer cursor via thought. In December 2021, trial participant 61 year-old Philip O’Keefe became the first person to make a social media post using an implanted BCI, tweeting the traditional “Hello World”.
Implanting BCIs to the motor cortex through the blood vessel allows minimally invasive access to parts of the brain which have historically required open surgery and the removal of parts of the skull. Dr Oxley previously described the blood vessels as “the natural highway to the brain”.
Patients suffering from paralysis and who are interested in participating should discuss with their doctor. Details for the United States can be accessed at clinicaltrials.gov while Australians should monitor australianclinicaltrials.gov.
Beyond Stentrode’s support for paralysed patients Synchron has 60 patent applications including treatments for epilepsy, depression, Parkinson’s disease, pain, addiction, and non-medical applications.
Synchron is the first company to undertake a trial under the United States Food and Drug Administration’s (FDA) investigational device exemption. This permits companies to undertake assessments on permanently implanted brain-computer interface (BCI) rather than the short-term experimental settings that have been previously approved.
The FDA investigational device exemption was awarded to Synchron in July 2021 after the firm closed a $52 million Series B funding round led by Khosla Ventures out of Silicon Valley the month before. Synchron also received US$10 million from the United States government body, the National Institutes of Health.
Although its research and development facility is in Melbourne, the company is headquartered in New York. Professor Opie said this decision was made for a number of reasons, which includes the greater experience for taking commercialising technology in the United States.
“Australia’s certainly one of the world leaders in translating research from designs and concepts through the first human trials and clinical studies. America on the other hand is very good at doing the next step and taking things through a regulatory pathway from the first human trials to a product that can be distributed and provided to patients by their physicians and caregiver.”
He also added that the United States has a much larger patient population. There are around 5 million patients in United States afflicted with paralysis.
Deputy director of the Sydney Institute of Criminology and academic fellow at the university of Sydney law school Dr Allan McCay sought to highlight the importance of balancing ethical questions about mental rights when developing neuroetch and the pressing needs of patients now.
“The one thing to I think one thing to bear in mind with all of this, is that there are great sort of ethical issues around neurotech but there’s also a sort of ethical imperative to address the conditions of people who have serious neurological conditions and need therapeutic devices,” Dr McCay said.
In particular, Dr McCay raised questions regarding the ethics of Elon Musk’s Neuralink technology which may involve neurological enhancement. Further taking a more general view of neurotech development, he noted future devices that collect brain data may compromise mental privacy and be used in conjunction with other personal data as a tool for manipulation. Although he hastened to add that there is no threat of manipulation through the Stentrode device.
Since its foundation in 2016, Synchron has raised a $97 million including $1 million from the United States Defense Advanced Research Projects Agency to complete a proof of concept and $2.2 million Australian National Health and Medical Research Council. Stentrode, which has been in development at the University of Melbourne since 2011, was recognised in TIME Magazine’s Best Inventions of 2021.
Do you know more? Contact James Riley via Email.