Brisbane-based vaccine patch company Vaxxas is developing the first room temperature-stable needleless vaccine for one of the world’s most common cold-like diseases, weeks after launching efforts to raise $100 million.
On Tuesday, Vaxxas announced it had received a global licence from the United States National Institutes of Health (NIH). The company will now prepare for the first clinical trial for a room temperature-stable needleless vaccine for Respiratory Syncytial Virus (RSV).
Vaxxas chief executive David Hoey said the “Vaxxas needle-free technology to eliminate the need for refrigerated distribution and enable self-administration”.
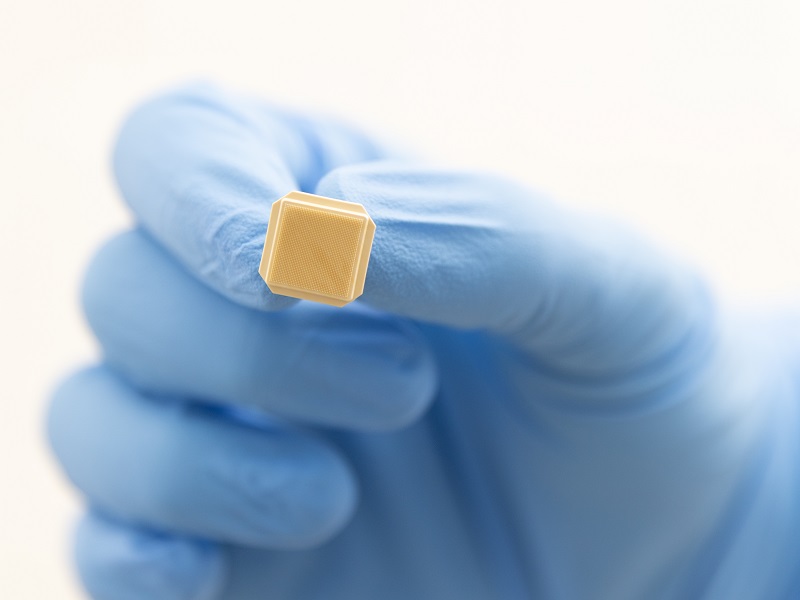
The University of Queensland spinout has licensed a vaccine antigen candidate from the NIH for use in its novel high-density microarray patch vaccine technology.
“Published preclinical results show the potential immunogenic advantages of this antigen candidate as the basis for an RSV vaccine to provide robust and durable protection,” Mr Hoey said.
While generally not a severe infection for healthy adults and children, the number of hospitalisations related to RSV each year is estimated to be 3.6 million, while the number of deaths is more than 101,000. It is the leading cause of hospitalisation for infants in the US.
In Australia, “almost all children experience at least one RSV infection within the first two years of life”, according to the National Centre for Immunisation Research and Surveillance Australia, a government-funded research centre.
The centre recommends that pregnant women receive immunisation, with the intent of protection being passed to the unborn infant, as well as elderly people from the age of 75 or in some cases 60.
The world’s first RSV vaccines, developed by pharmaceutical giants GSK and Pfizer, were approved in the US and Europe in 2023.
Earlier this month, it was reported that Vaxxas hopes to raise $100 million to fund late-stage clinical trials for its influenza and COVID-19 vaccine products and to launch the RSV clinical trial.
Vaxxas has so far completed five phase one clinical trials, involving more than 500 participants each, including a COVID-19 vaccine candidate, a HD-MAP delivered flu vaccine, and a measles and rubella vaccine.
The company has also received $43 million in funding from the United States Biomedical Advanced Research and Development Authority to run its first phase one clinical study that is ‘investigational new drug’ enabled, a key step in getting approval from the US Food and Drug Administration.
That trial involves a pre-pandemic influenza vaccine involving 258 participants in Australia.
Do you know more? Contact James Riley via Email.